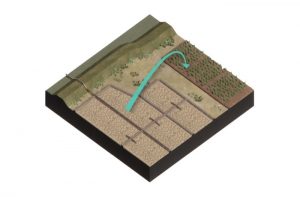
Chemical ripening
As a result of increased aeration fostered by physical ripening, oxidation of soil components that are only stable during reduced condition in the absence of oxygen will take place. This process changes the ionic composition and concentration of the soil. It mediates weathering of less stable minerals but also the formation of new minerals (Pons & Van der Molen, 1973).
The oxidation of sulfur is one of the main chemical ripening processes. The visible effect of oxidization of sulfur can be recognized by a change in color of the sediment from black to brownish or yellowish gray colors. The change of color is primarily the result of oxidation of iron-sulfide (FeS) to iron-hydroxides (Fe(OH)3). The redox potential is therefore a good quantification of the degree of oxidation of the soil (Gliński & Stępniewski, 1985). The redox potential of soil and/or water systems are a measure of the electrochemical potential or electron availability within these systems.
Another outcome of oxidation is a decrease in Ph due to the formation of sulfuric acid. In the absence of a buffer this can lead to the mobilization of heavy metals like copper and zink (Calmano, Hong, & Förstner, 1993). Contrarily, metals like iron and magnesium experience lowered mobility during low Ph, which dictates the solidification of Iron-hydroxides (Singh, Tack, & Verloo, 1998). This is important for the ripening process as in some cases mobility of certain ions is not always wanted because of ecological constraints. In order to avoid leachate flowing into restricted areas, a project manager should know in which chemical ripening state the sediment is.