Get Started
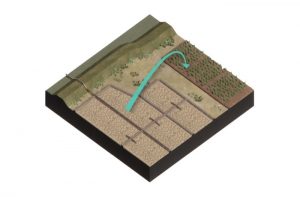
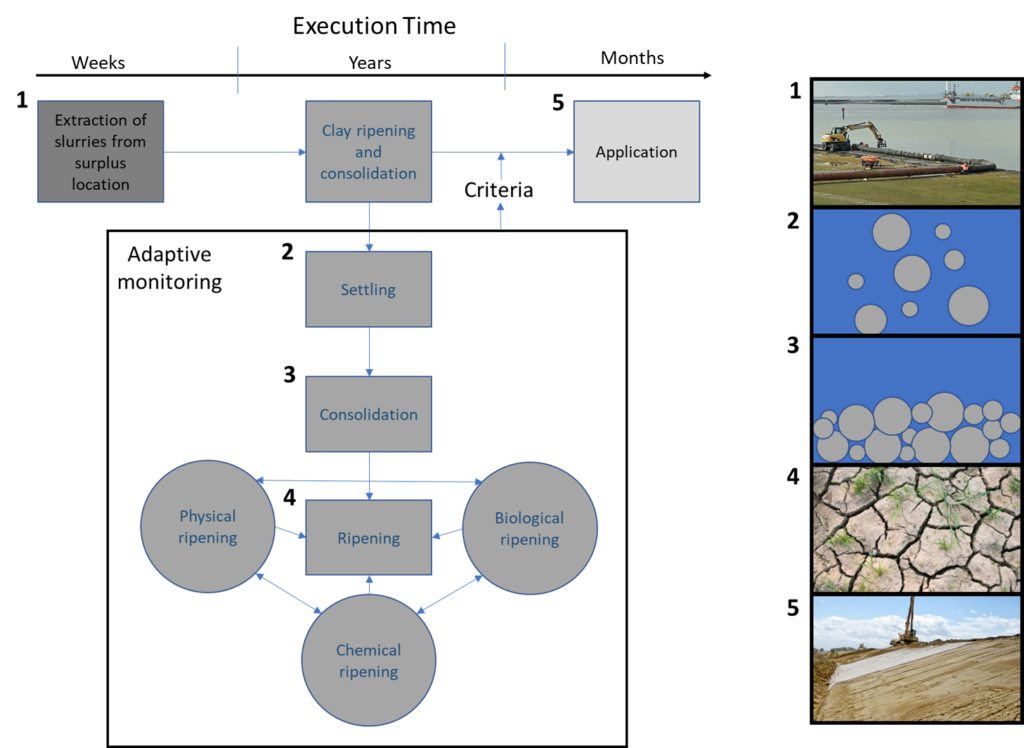
Before diving into various steps that guide you through the technical aspect of implementing a ripening and consolidation project, the most important natural processes will be explained in this section. The processes will be discussed sequentially from the suspended state until the formation of a ripened soil. Mud undergoes three main phases: 1) settling, 2) consolidation, and 3) ripening.
Settling
Once mud enters calmer waters or an upland basin for ripening, the particles can settle following Stokes’ law: the particle size and density dictate settling velocity is (Stokes, 1850). The smaller the particle size and density, the smaller the settling velocity. Thus, the settling process is faster if particles increase in size, which can occur if particles are non-cohesive (fine material: (≥20%) of clay minerals).
Flocculation
If settling time allows particles to interact with each other, destabilized clay particles can congregate into larger agglomerates (flocs) by a process which is called flocculation (Bratby, 1980). Flocculation takes place before and during settling. Flocculation befalls because of the attraction between the negative face chargers and positive edge charges of the clay particles.
Hintered settling
If clay particles are very close together and form slurries, particles cannot fall without hinderance. We call this hindered settling. During this kind of settling, settling occurs at reduced speed due to interactions with neighboring flocs/particles, which hinders (or decreases) the settling velocity of an individual floc/particle. The larger the concentration of solids in a suspension, the lower the settling velocity of particles. During this phase, the lowering of mud-water interface occurs with a lower speed than in the settling phase.
Gelling concentration
The end of hindered settling phase is characterized by the gelling concentration (Cgell) at which a space filling network (“skeleton”) develops, which means that all flocs/particles are considered to be in contact with each other, leaving no possibility for further settling. In other words, the gelling concentrations is defined as the concentration at which the bed begins to exist. This moment is characterized by the transition from hindered settling to the first phase of consolidation (Meshkati Shamirzadi, Wichman, & Hansen, 2018). This moment can be recognized by a sudden decrease in the descension rate of the mud-water interface.
Consolidation
Consolidation is caused by the overburden pressure resulting from the weight of overlying sediment (Terzaghi & Peck, 1967). During the consolidation phase, the flocs rearrange themselves into a denser structure. The pore water between the particles is being expelled by the overburden pressure. If the soil is more permeable, water extrusion is faster which positively impacts consolidation. Consolidation can be divided into two phases.
Phase 1
The first phase of consolidation starts after the gelling concentration is achieved. In this case, the lowering of mud-water interface (dewatering) is governed by permeability and is much slower than of in (hindered) settling phase. Hence, the transition between both phases can be recognized from the sudden change in slope of the curve of the interface height with time.
Phase 2
The second phase of consolidation starts, when deformations (e.g. reduction in mud-water interface) are even smaller than during the first phase of consolidation. In this phase, effective stresses dominate the consolidation process.
Ripening
If evaporation exceeds the outflux of water by consolidation, the ripening phase starts. Ripening is a soil formation process that inevitably modifies waterlogged sediments into soil by desiccation and structure development (Pons & Van der Molen, 1973; Vermeulen, Grotenhuis, Joziasse, & Rulkens, 2003). In order to grasp the essentials of how to ripen dredged sediment, a background of the three main subprocesses of ripening of clay, that are co-occurring simultaneously, is needed:
Physical ripening
Fine sediment is being converted to a more compact material beyond recall. This is done by exposition to drier hydrological conditions than those it can maintain equilibrium with (Rijniersce, 1983). Physical ripening makes fine sediment more aerated and permeable. The newly materialized soil beholds physical properties which depend on the new hydrological conditions. When consolidation decreases the water content and permeability, the soil shrinks. Shrinkage induces crack formation of the soil, hence increasing the permeability slightly. More detailed information on physical ripening.
Chemical ripening
As a result of increased aeration fostered by physical ripening, oxidation of soil components that are only stable during reduced condition in the absence of oxygen will take place. This process changes the ionic composition and concentration of the soil. It mediates weathering of less stable minerals but also the formation of new minerals (Pons & Van der Molen, 1973). More detailed information on chemical ripening.
Biological ripening
Biological ripening encompasses the effect of all kinds of soil flora and fauna, including larger and microscopic forms of life, but excluding direct activity of humans (Pons & Zonneveld, 1965). The main agents of biological ripening are microorganisms, worms and vegetation. More detailed information on biological ripening.